For e-commerce businesses
We all shop online. Have we observed how an Amazon site throws up multiple recommendations when we are searching for a product or have already added something to the cart? That’s probably one of the most common use cases of an AI engine giving customized recommendations based on the consumer’s current and past searches. It helps e-commerce businesses increase cart size. At Navikenz, we recently built solutions for a travel tech company around their cloud-migration, app modernization, and providing customized and personalized travel packages to its customers.
However, AI can help much more than this. Through AI/ML models, one can understand why customers are abandoning carts, and companies can take corrective actions. AI can also plan for your last-mile delivery. AI-powered delivery scheduling algorithms can optimize delivery routes, consider customer preferences, and time windows, and dynamically allocate resources to ensure on-time deliveries and reduction in your overall delivery costs.
For businesses that require warehouse management
Efficient warehouse operations are critical for D2C businesses to manage inventory, fulfil orders, and meet customer expectations. AI-powered warehouse optimization algorithms can analyse data on inventory levels, order volumes, and product demand to optimize warehouse operations. For example, these algorithms can automatically allocate storage space, optimize picking routes, and predict demand to prevent stockouts and overstocks. AI can also be used to automate warehouse processes, such as inventory counting, packing, and sorting, improving operational efficiency and reducing human errors.
Gaming companies
The creative and research team can analyse content and its performance through AI to develop more palatable and innovative games for its consumers. AI can decode the elements in video content, including the characters, emotions, story arcs, and more. It can then go on to provide a much deeper analysis of the gamer’s behaviour to produce more relevant content.
Education
AI can assist for special needs of students – for example, making the content audio for a visually impaired student. AI is also being used currently in high schools to detect plagiarism. With the advent of tools like ChatGPT, students will increasingly use it for even writing a simple essay. AI can help grade even abstract assignments like essays.
Navikenz can provide comprehensive AI/ML solutions tailored to the specific needs of D2C businesses, helping them leverage technologies to optimize their operations, enhance customer experiences, and gain a competitive advantage in the market.
Don’t miss out on the benefits of AI for your D2C business. Contact us at [email protected] to learn more about how our AI/ML solutions can help optimize your operations and improve customer experiences.
Having observed the role of Enterprise Architecture throughout my career, from the late 1990s to the current era of cutting-edge enterprise demands in the field of AI in 2023, I can confidently affirm the substantial evolution and growing significance of Enterprise Architecture. I have seen it empower organizations to successfully undergo digital transformation, witnessed enterprises achieve their strategic goals, and bridge business objectives and IT.
Today, in the era of AI, Enterprise Architecture transcends addressing an organization’s current business objectives and extends towards anticipating the organization’s future requirements in an increasingly digital and data-driven landscape.
At its core, Enterprise Architecture has always been the practice of aligning an organization’s business strategy and goals with its information technology infrastructure. It has always focused on blueprinting the design and implementation of technology solutions that support an organization’s objectives.
So, how has it evolved to meet the challenges of the current era of AI?
How has it become all the more significant now?
How can Enterprise Architecture enable Enterprises to adapt to AI?
Before jumping to perfect answers that even a GPT can provide, I would like to rewind my memory lane for a more pragmatic answer, and I would like to do this in two parts:
Part 1 Evolution of Enterprise Architecture in the Era of AI: A Career Journey and Practical Insights
Part 2: How Navikenz Enterprise Architect team helps our customers adopt AI?
Part 1: Evolution of Enterprise Architecture in the Era of AI: A Career Journey and Practical Insights
In 1997, while I was busy building Dynamic Link Libraries (DLLs) and distributed com solutions during the .com boom, the origin of Enterprise Architecture began to rise and gain traction with the emergence of The Open Group Architecture Framework (TOGAF) and Full Environmental Assessment Framework (FEAF). When the field of information technology provided the capabilities to automate business processes, organizations began to invest heavily in technology. The need for organizing information systems, implementing distributed computing systems, and enabling communication between disparate systems were the prime problems that Enterprise Architecture focused on solving.
The SaaS product I was working on in 2001 demanded a technology solution that could scale rapidly, handle large volumes of traffic, and provide a seamless user experience. Enterprise Architecture enabled that with grace. It provided a way to ensure that the technology infrastructure was aligned with the business goals and could support the demands of the online marketplace.
Then came the emergence of mobile devices. On top of the core mobile solution, my customer needed to develop a consistent user experience for their banking customers across different platforms and integrate with existing IT systems. Very soon, the need to scale for rapid mobile adaptation became a growing need.
Enterprise Architecture played a critical role in addressing all these needs by providing a framework for developing and implementing responsive and adaptive solutions to the needs of the mobile era. It also provided a standard for integration solutions that could handle the complexity of integrating mobile applications with back-end systems. By taking a holistic view of the organization’s IT architecture, Enterprise Architecture helped organizations to develop mobile solutions that could leverage existing systems and data, rather than creating siloed solutions that would be difficult to integrate with the rest of the IT infrastructure.
Cloud computing also began to gain traction during this time, and Enterprise Architecture had to adapt to support the migration of enterprise applications to the cloud. The challenges posed in cloud transformation were multifaceted for the different organizations I had consulted. While legacy systems were rusted enough to touch and demanded a complete re-architecting to work in the cloud, data migration was altogether an uphill task with the volume and complexity involved. Security posed a lethal combination of challenges including mindshift adjustment, compliance and regulatory, and technology solutioning.
My guiding lights during several cloud migration programs were the fundamental Enterprise Architecture principles:
- Alignment with business goals
- Modular design
- Standardization of technology platforms and tools
- Prioritizing security and compliance from design
- Agility to respond to changing conditions
In Part 2 of this blog series on the Evolution of Enterprise Architecture in the Era of AI, we will delve into the practical insights and experiences of the Navikenz Enterprise Architect team. With the rapidly changing landscape of technology and the increasing demands of businesses to incorporate AI, the role of Enterprise Architecture has become even more significant. In this blog, we will explore how Navikenz helps its customers adapt to the challenges and opportunities presented by AI through the lens of Enterprise Architecture. We will examine the approach, tools, and methodologies used by Navikenz to enable successful AI adoption and integration in organizations.
Pharmacovigilance is increasingly dealing with a variety of rapid technology, regulatory, environment and business changes. The safety and efficacy of medications remain a key priority for healthcare providers, patients, and pharmaceutical companies. In this era of increasing automation, the use of innovative technologies and processes to ensure drug safety and pharmacovigilance has continually grown in importance. Automating drug safety and pharmacovigilance processes can lend an edge to organizations by reducing the burden of manual methods, providing better scalability, improved operational efficiency, and minimizing the risk of human errors. We are witnessing a few key trends across the industry in recent times.
Current Industry Trends towards smart case processing
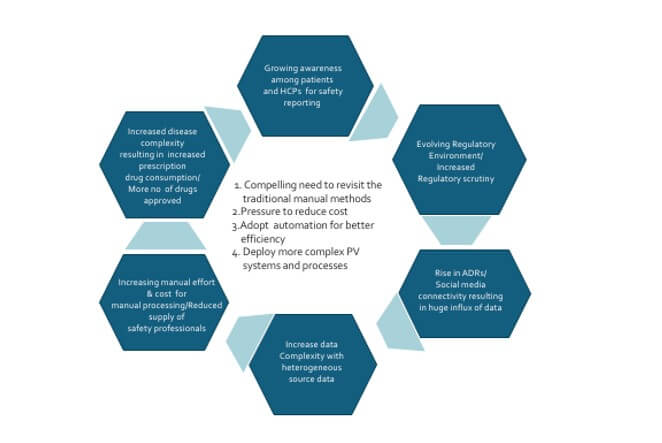
- As the cost of manual labour rises, there is an increased adoption of digital solutions to improve operational efficiency and reduce operational costs. More investments are seen in the development of pharmacovigilance tools and technologies. This can include the development of online databases, mobile applications, and software tools to facilitate the process of drug safety monitoring. In addition, digital solutions make it easier to analyse the data and extract insights that can help organizations optimize operations. Digital solutions provide several benefits for drug safety and pharmacovigilance
- Drug safety and pharmacovigilance activities are becoming increasingly automated when it comes to analysing large datasets, gathering adverse event reports, and more. Automation trends are rapidly changing the way drug safety and pharmacovigilance processes work, making them more efficient, effective. and less resource intensive
- Automation is reducing the time, effort and cost associated with data entry, document review and other manual processes. Automation can help streamline the process and reduce the risk of errors, by eliminating the need for manual data entry and providing a more detailed view of the data. It helps to improve compliance rate and data accuracy. It reduces resources needed for manual entry and review, also provide reliable results at faster pace. Automation can also simplify processes by making it easier to track and process large amounts of data. Additionally, automation can enable real-time alerts to be sent to quickly identify potential safety issues.
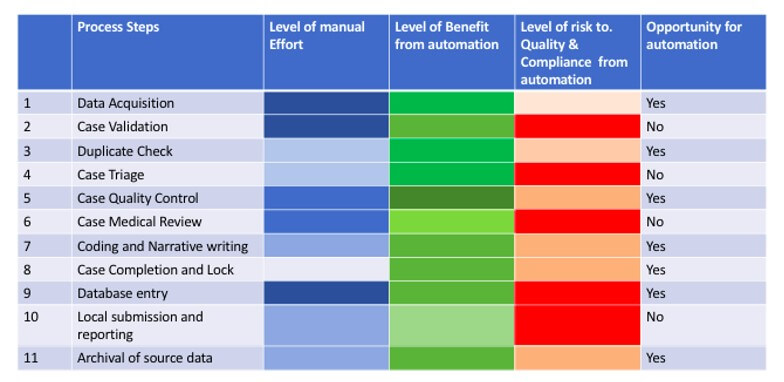
Though automation is highly beneficial, Intelligent automation is being explored across each process step within pharmacovigilance area to ensure compliance to the regulatory requirement and reduce risk due to automation. It is essential to break down and analyze each process step in terms of manual effort, automation potential, benefits, and risks to data quality and compliance. Once this has been done, this automation can be generically adopted by pharmacovigilance functions in various organisations despite organisational variations in specific process steps.
Introducing Navi-CADE:
In the age of digitalization, business processes are being automated using the latest technological advancements. The motivation behind these efforts is to make these processes agile and efficient. One of the business processes is involved in handling and processing large number of documents with different layouts, purpose and source. These can be structured documents like Loan Application Form, Insurance Claims, Tax Returns or unstructured like handwritten doctor prescription, legal contracts, scientific publication, and others.
Keeping in view of the wide spectrum of industries which need document processing solutions, we have developed Navi-CADE. Navi-CADE is a solution to search and process documents, using stack of extendible and reusable microservices, which can handle entire lifecycle of document processing, right from document ingestion to extracting domain entities and building ontologies. It is designed to handle documents both structured and unstructured across industries like Insurance, Taxation, Pharma and Lifesciences.
In addition to AI (Artificial Intelligence) services, we leverage custom built deep learning models like BERT for domain specific intelligence. For example, BioBERT is used to get in-depth domain understanding of medical text using wide spectrum of medical literature to provide contextual understanding using UMLS and other such resources. This will enable faster and efficient analysis of adverse event reports in case processing or further contributing towards case evaluation to detect a possible signal in pharmacovigilance. Another use case can be in loan processing where incoming loan application can be evaluated and preliminary evaluation can be done to check if loan can be approved or not based on the entities extracted from the documents and compared as per bank standards.
The key drivers of this solution are – reusable functionality, composing each individual functionality into a microservice, extending the functionality based on the business requirements, adopting latest tools and technologies to build an agile set of services and services which can be deployed quickly across different infrastructure setting.
An example of the real-life use case we have developed using this framework is – Case Processing in Pharmacovigilance. In this use case, we have used this framework’s services to address processes starting from case ingestion through various channels like emails, database, and others, performing translation of the documents to English, extracting vital information from the documents to identifying key entities which supports case evaluation process and further extending their knowledge with the use of external medical journals and databases.
To understand how Navi-CADE can be used in current business processes, we need to know about the challenges being faced by the industry in document processing space. These challenges are –
- Quantum of documents being generated in today’s digitalized world can overwhelm the legacy systems of document processing
- Diversity in the source and structure of these documents requires a solution to understand the document layout and then process it accordingly.
- Risks involved in some of the highly regulated industries like Insurance add another layer of complexity for the document processing solutions.
Keeping in mind these industry challenges, we have designed Navi CADE in such a manner that it gives flexibility to the business to deploy it as per their processes. Deployment of this framework involves –
- Defining the use case for which we would like to deploy this solution like processing loan applications or case processing in Pharmacovigilance
- Once we have identified the use case, we need to map the flow as per our business process
- We will define the possible sources of documents like in-house database, email server or cloud storage etc.
- Next, we define parser for our document based on the purpose, structure, and domain of the incoming documents. This is crucial to ensure reliable information extraction from the documents
- Then as per normal business process, along with our main form, we will have supporting documents like identity documents, address proofs, diagnostic results etc. We need a system to automatically identify and categorize these documents accordingly using custom document classification models.
- Once we know the category of each document, we can extract information and gather entities from the document for further deeper analysis.
- Finally, we would like to store all the results in a database and generate some summary report.
All these tasks can be performed by individual microservices, developed using underlying AI/ML services provided by AWS (Amazon Web Services) and this entire operation is orchestrated by an orchestrator which ensures all the tasks are performed sequentially.
The overall architecture of Navi-CADE can be categorized into three main service categories:
- AWS Services like Textract, to extract information from the documents, translate to perform translation, comprehend medical, to extract entities from the document information and map them further to external medical journals and databases using MEDRA coding
- Core Services perform generic document processing tasks using AWS Services mentioned earlier. These tasks are Ingestion of documents from diverse sources, performing translation, document classification, entity extraction and then using MEDRA cording to tag all the information to a standard medical term.
- Domain Services involves incorporating certain domain specific capabilities using state-of-the-art transformer models like BioBERT, LegalBERT and others. It also includes orchestration and pipeline capabilities.
Navi-CADE has been developed to address some of the key challenges faced by the industry like –
- Exponential Growth in the Documents generated at a particular point of time in an organization. With the rapid digitalization, the quantum of document processing requirements has made traditional processing capabilities ineffective and inextensible. The variety and veracity of documents makes processing even more complex and challenging.
- Documents are of several types and structure. Some are handwritten whereas some are attached with email files or could be stored in a datastore. Solution should be capable of handling documents of diverse design and coming from various sources.
- As different documents have different layouts it makes it extremely laborious to understand the structure of every document and then process it. Manual tasks are incapable of handling this complexity as they require documents to be in pre-defined structure only.
- Documents generated in highly regulated industry such as FSI and Pharma need to be handled with extra care due to the risk involved. ML (Machine Learning) / DL can play a key role in identifying such risks in the form of Terms, Contracts, Legal etc.
Navi-CADE can address all the above-mentioned challenges with the use of AI/ML services and automating and augmenting critical document processing tasks in an organization.
To discover more about how Navi-CADE and other innovative technologies can optimize your drug safety and pharmacovigilance processes, please reach out to us at [email protected]
For the past few decades, most companies have kept data in an organizational silo. Analytics teams served business units, and even as data became more crucial to decision-making and product roadmaps, the teams in charge of data pipelines were treated more like plumbers and less like partners.
In the current age, things have become different. The most forward-thinking teams are adopting a new paradigm: treating data like a product or DaaP. Fundamentally, Data as a Product is a concept, or methodology, about how data teams can create value in their organizations. Adopting an organizational approach of treating data like a product isn’t just a buzzworthy trend in the data industry. It’s an intentional shift in mindset that leads to meaningful outcomes: increasing data democratization and the ability to self-serve, improving data quality so decisions can be made accurately and confidently, and scaling the overall impact of data throughout the organization.
While approaching data as a product, organizations should think about the entire value chain from ingestion to consumer-facing data deliverables & what are the KPIs against which they want to measure the success of their data products. One must prioritize data quality and reliability throughout the data lifecycle. Data teams are working to find processes and systems that help them advocate for the importance of data on a wider organizational level. There are two broad mandates that data teams tend to get formed with:
- Provide data to the company
- Provide insights to the company
The job of the data team is to provide the data that the company needs, for whatever purpose, be it making decisions, building personalized products, or detecting fraud. This might just sound like data engineering, but it’s not. Many data teams are adopting KPIs related to data quality, such as calculating the cost of data downtime—times when data is partial, erroneous, missing, or otherwise inaccurate—or by measuring the amount of time data team members spend troubleshooting or fixing data quality issues, rather than focusing on innovations or building new data products.
Companies can assess their current state of data quality by mapping their progress against the data reliability maturity curve. Briefly, this model suggests there are four main stages of data reliability:
- Reactive: Teams spend most of their time responding to fire drills and triaging data issues—resulting in a lack of progress on important initiatives, an organizational struggle to use data effectively in their product, machine learning algorithms, or business decision-making.
- Proactive: Teams collaborate actively between engineering, data engineering, data analysts, and data scientists to develop manual checks and custom QA queries to validate their work.
- Automated: At this level, teams prioritize reliable, accurate data through scheduled validation queries that deliver broader coverage of pipelines. Teams use data health dashboards to view issues, troubleshoot, and provide status updates to others in the organization. Examples include tracking and storing metrics about dimensions and measures to observe trends and changes or monitoring and enforcing schema at the ingestion stage.
- Scalable: These teams draw on proven Dev Ops concepts to institute a staging environment, reusable components for validation, and/or hard and soft alerts for data errors. With substantial coverage of mission-critical data, the team can resolve most issues before they impact downstream users. Examples include anomaly detection across all key metrics and tooling that allows every job and table to be monitored and tracked for quality.
We, at Navikenz, have been helping organizations to move from stage 1 to 4 successfully. Also, over the past few years, companies have gotten wise to this, and have started using a different model (in consonance with DaaP) — Data as a Service. Will talk about DaaS in upcoming blogs.
Interested in transforming your organization’s approach to data? Contact us at [email protected] to learn how we can help you move from reactive data management to scalable data reliability. Let Navikenz be your partner in creating a culture of data-driven decision-making and innovation in your organization.
Age of craftsmen -a Standardization and process driven manufacturing -a Personalization at scale
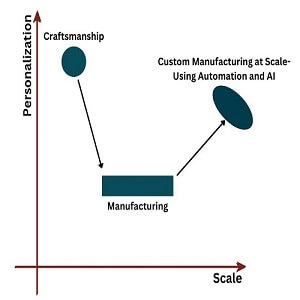
We talk about three core values at Navikenz – Celebrate differences, Evolve Everyday, and Technology Second Outcomes First. Our understanding of these values is usually contextualized around what these mean to us as employees, and how we intend to conduct ourselves. But in this note, I want to explore what our values mean in the context of how we work with our customers
Celebrating differences in our customers implies that all our solutions are uniquely crafted to serve the specific requirements that our clients have from us. This means that we do not serve our clients with a standard cookie-cutter template, and we do not force-fit pre-existing solutions to meet unique client needs. In some ways, we approach our solutions in the same way a master craftswoman does – pulling from her deep reservoir of experiences, and utilizing a host of tools and templates to create something that is uniquely suited to her customer’s needs.
For the craftswomen, this comes naturally – over many years of experience, she is able to remember her learnings from previous work, and has evolved a host of shortcuts and personal methods that allow her to create high quality and unique solutions each time. But this way of working creates a natural limitation of scale – she is a single person and can only do so much. Others cannot access the information in her mind and create similar solutions. So, she will only produce as much work as a single (albeit highly efficient) person will do.
At Navikenz, we aspire to provide this same level of customized service at scale. We have given ourselves an ambitious growth plan which will require us to build dozens of ‘master craftspeople’ in a short amount of time. How can this be achieved? Most other technology service organizations simply go into the market and hire a vast number of specialized people at great cost and effort. We know that as a young company, we have neither the time, nor the financial depth to do this. Moreover, we have hired many smart and ambitious young people, who are ready and willing to serve our clients, but lack the experience that is available with ‘masters.’
We need to enable our young people with a platform where the experiences and learnings of all Kenziens can be pooled together into a common storehouse of learnings and experiences. This ‘Organizational experience,’ which would be accessible to all Kenziens, can become far deeper, and broader than anything that a single craftsman can ever hope to achieve. By providing the tools for Kenziens to access these learnings, we will be able to help all Kenziens to rapidly evolve into master craftspeople – provide high quality, custom solutions to our clients AT SCALE.
Importance of real-world data for Pharmacovigilance
Drug safety is of utmost importance when it comes to patient care. Even though clinical trials are carefully designed and conducted, they still have limitations. Patients with comorbidity, pregnant women and children are often excluded from clinical trials, which can limit the generalizability of the results. The clinical trials do not reach the power to identify all the possible adverse reactions (rare & very rare) with the drug. Hence there is limited information on the safety and efficacy of a medicinal product at the time of receiving marketing approval. Additionally, drug-drug interactions (DDIs) may not be fully known or understood during clinical trials.
This is where real world data comes in.
Real world data can provide valuable insights into the safety and effectiveness of drugs in the general population, including those with comorbidities and potential DDIs. By analysing real world data, we can better understand the risks and benefits of drugs in real-world settings, leading to better patient care. Real world data can be collected from various sources, such as electronic health records, claims and billing data, and patient-generated data. This data can be used to identify trends and patterns in drug safety and effectiveness, as well as inform regulatory decisions. While clinical trials are still important for drug development and approval, real world data can complement clinical trial data and provide a more complete picture of a drug’s safety and efficacy. Therefore, it is crucial to continue to prioritize the collection and analysis of real-world data for drug safety.
Ever changing sources of data and complexity
The current challenge of pharmacovigilance is two-fold. First, there is a lack of consistency in how safety and efficacy data is collected, analysed, and reported. This leads to confusion and can lead to medical errors. Second, the sheer volume and complexity of data available makes it difficult to separate out the signals that indicate potential health issues. In the future, pharmacovigilance will become increasingly complex as more data sources are available, from electronic medical records to mobile health tools. With this complexity comes a need to develop better methods of integrating and analysing data, as well as developing systems that enable predictive analytics. As such, it is essential that healthcare providers and regulatory bodies remain abreast of the current and future needs of pharmacovigilance.
Healthcare information systems are generating more patient-level data than ever before with significant rise in prescription drug consumption and number of patients taking prescription drugs. This high influx of data must be managed and analysed if drug safety is to be monitored effectively. The use of non-traditional data sources such as digital and social media for pharmacovigilance is on the rise with advancement in technology and ease with which the population is accessing these technology tools. Data is getting generated from sites such as Twitter, Reddit, Facebook, YouTube, and Instagram, among other sources. Social media platforms like Twitter and Facebook have become a popular forum for patients and consumers to share their experiences with products and services. This includes sharing of information and online conversations about adverse events, and as a result, social media has become an important source of data for pharmacovigilance and drug safety. These data sources can provide valuable insights about patient safety and the potential risks associated with certain medicines. New data sources can be accessed to capture safety signals more accurately and timely that may otherwise be missed. New sources of patient-level data, such as electronic health records, mobile health applications, and wearables, provide a more holistic view of drug safety and patient demographics.
The emergence of digital and social media has presented the pharmacovigilance function with a range of opportunities as well as challenges. As technology advances, so does the complexity and volume of available data sources which is making it difficult to fully understand how they can be best leveraged to support patient safety. Existing data mining and data analysis techniques must be upgraded to be able to handle the new types of data. Some of the challenges associated with social media data on adverse events include:
- Difficulty in collecting relevant data from disparate sources
- Identify and separate safety signals from the vast amount of data
- Ensuring data accuracy and quality
- Developing appropriate analytical tools to interpret
- Identifying trained resources to manage this level of volume and data complexity
- Ensuring patient privacy and confidentiality
- Ensuring compliance to regulatory requirements
Despite these challenges, social media data remains a valuable resource for monitoring adverse events and identifying potential safety issues. It is important for regulators and pharmaceutical companies to continue to explore ways to leverage this data while also addressing the challenges it presents.
Additionally, these data sources often include information that is changing very rapidly. Pharmacovigilance teams must be equipped with the necessary algorithms and tools to ensure the data is both accurate and up to date. At the same time, they must also ensure they continue to comply and adhere to local and international regulations with respect to data security and privacy.
The increased availability and complexity of data is driving the need for better processes and infrastructure to support the efficient and timely use of data sources. The development of an integrated approach, with the focus on safety intelligence, data integration, and process automation, is essential for pharmacovigilance to be as effective as possible. Companies must have the right technology and processes in place to ensure data accuracy and up-to-date information. Additionally, data privacy and security must be a priority, to abide by the respective regulations. By understanding and effectively leveraging the data sources available, pharmacovigilance teams can best support patient safety.
Pharmacovigilance in the Big Data Era
Traditional methods of pharmacovigilance are no longer sufficient to handle this unprecedented growth in data volume. With millions of drug products now available in the market, the sheer volume of data coming from disparate and heterogeneous sources requires more advanced approaches to pharmacovigilance. The data needs to be converted from unstructured to structured form before subjecting it to the different data mining and analysis techniques.
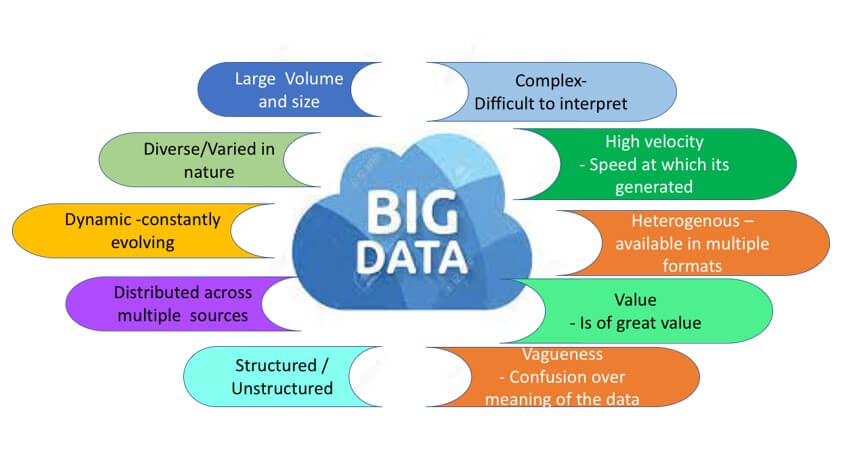
Recent technological advances and data mining techniques have enabled the development of innovative pharmacovigilance strategies to identify potential safety signals. To ensure the safety of drug products, the pharmacovigilance field has embraced the use of big data techniques to analyse large volumes of information. AI (Artificial Intelligence) and machine learning technology is widely employed to collect and analyses large and complex datasets. By leveraging advances in artificial intelligence (AI) and machine learning (ML), pharmacovigilance teams can now detect safety signals much sooner, more efficiently and more accurately than before. This allows much quicker action on the usage of drugs and prevents harm to the larger population. Additionally, the use of predictive analytics can help anticipate adverse events before they occur and provide insight into how drugs interact with other treatments.
The use of Artificial Intelligence (AI) and Machine Learning (ML) can bring major transformation in pharmacovigilance and drug safety signal management. AI and ML can be used to reduce the manual efforts spent on a wide range of drug safety activities and improve the results on signal detection and management. AI-driven systems can be used to automate and improve the signal detection process by identifying information from large sources including spontaneous reporting system (SRS) databases, Electronic Health Records (EHRs), and other structured and unstructured data sources. This data can provide a more detailed picture than what is provided by manual methods, drastically improving the safety signal detection process. AI and ML can also be used to alert medical professionals to potential safety issues faster, while also providing greater insights into the effectiveness of treatments. Additionally, AI can be used to identify potential drug-drug interactions and provide personalized predictive analytics. AI-driven systems can even be used to monitor safety over time and quickly identify potential safety issues in future. All these advances will be invaluable in ensuring the safety of patients and efficiency of the drug.
Starting your career as a young intern can be both exciting and nerve-wracking. You might be feeling overwhelmed with the new environment and expectations, but don’t worry, as you are not alone.
As you embark in your career there are many things that I would love to share with you, things I have seen, learnt and heard. And I will. But each person learns in their own way and charts their own path. The most important thing is to determine what path you want for yourself and how you want to walk that path.
Below are some pieces of advice to help you navigate your way as you start your professional journey.
- Learn Patience: Things take time. Rome was not built in one day. It takes 10,000 hours of practice to learn anything with competence. Focus on learning, focus on investing in the future. If you want the future to arrive yesterday, it might not ever arrive. Everyday think about “what did I learn” and never forget that key to a good life is ‘lifelong learning’.
- You might be the smartest person in the world, but if you cannot work with people well, if you cannot communicate, cannot write, and cannot speak in a way that conveys your thoughts, either nobody will know, or nobody will want to know anything from you. It is not what you say, but much more often, how you say it, that matters. How are you improving yourself so that you are more likable, and people are more willing to listen to what you say? Think about that.
- Integrity is what you do when nobody is looking. Whenever you are in doubt on whether you are doing the right thing, think “if my mother, brother or friend were here, would they support what I am doing?” One of the best pieces of advice I got from a boss of mine many years back was ‘I hate small lies’. There is no reason to lie. Sometimes not lying takes a little bit more effort. Take that effort. Do not lie. Small lies lead to larger lies and there is no end after that.
- Do your job. Do your job well. Then help your teammates do their job better, and then see if you can help your boss do his or her job better. Then help your boss’s colleagues do their job better. You will have a great and successful career.
- Always remember that you have multiple roles in your life. You are a person working in a company, you are also a Son/Daughter/Brother and a Sister. A friend. Your responsibility is to all your stakeholders in society and how you do one thing is typically how you do everything. Do not forget to call you mother and father regularly, thank them for what they have done. As we get busy in life, we tend to forget those who made us possible. Don’t fall in that crevice.
- In the first 15 years of one’s career people tend to do enormous damage to their health. You are young and feel you are invincible. But the way you look after your health now will determine how your body looks after when you are 40 years old. Age 40 seems like a long way off, but it will come in a flash. And you cannot relive the past. Eat well, exercise, mediate. Nourish the soul and the body. It will help you lead a productive life ahead,
- In every interaction learn something from those around you. There is always something to learn from in any situation. Also, in every interaction, give something. The easiest thing to give is ‘positivity’ and a ‘smile’. Make people feel better that they met you today. When you have a cup of Tea in a restaurant, look into the eyes of the server, ask for their name, and say thank you to them by name. When you get off an airplane, look into the eyes of the Flight Attendant and say ‘Thank you’ for taking care of you during the flight. You will be surprised how happy you will make them. We are privileged to be part of this world. Thank everybody who makes that possible.
- Life is not easy and throws challenges at us. We all feel we are more unfortunate than other people. Universally. That is not true. Every human being has challenges. Our misery is only our lack of appreciation of the misery of others. We are fortunate. Every day. Write a ‘Diary’ to yourself regularly. If possible, every day. You will be amazed at how that will help you manage the challenges that life throws at you.
- Remember ‘you can fool some people all of the time’, ‘fool all people some of the time’, but ‘you cannot fool all people all of the time.’ I think when we try to fool other people, we are only fooling ourselves. Do not be tempted with that. Be true to yourself.
- We all want success. You cannot get success for yourself unless you are intensely focused on the success of others. Success of your team, of your organization and of your customer. You will get success and happiness.
Starting your career as a young intern can be overwhelming, but it is important to remember that with patience, hard work, and a positive attitude, you can achieve great things. Always be open to learning, communicate effectively, and take care of yourself and others. And most importantly have a lot of fun. Enjoy what you do and do what you enjoy.
With these tips in mind, you are well on your way to building a successful career. Here’s wishing you all the very best that life has to offer !!
Recent trends in drug safety and pharmacovigilance have revealed that drug related adverse events are the major cause of death in the United States. Every year, an estimated 200,000 to 400,000 people die due to adverse drug reactions, which is more than stroke and diabetes combined. Approximately 2.7 million adverse drug reactions occur annually in the US, resulting in over 100,000 hospitalizations and more than 15,000 deaths. This makes ADRs the fourth most common cause of hospitalisation in the USA, behind heart attacks, stroke and pneumonia. There are several factors that contribute to America’s growing number of hospitalisations and deaths related to adverse drug reactions. These primarily include, poor patient compliance with medication instructions , lack of education on potential side effects , unnecessary prescribing of medications and increased use of polypharmacy, or combining multiple medications
What has changed ?
However in recent years the space of Pharmacovigilance been changing rapidly due to advances in technology and the influx of data. In the past five years, there have been many changes in the way the field is handled. As the range and scope of drugs being approved and being used expand, monitoring the safety of medicines and ensuring that they are being used correctly and as directed is becoming an increasingly complex task. With more and more number of drugs being available, pharmacovigilance practitioners must remain on top of the latest developments in order to ensure that patients are receiving the safest and most effective treatments available.
The total number of prescriptions dispensed in the United States increased from 3.9 billion in 2007 to 4.3 billion in 2012, according to the Centres for Disease Control and Prevention. This represents an increase of 11%. This can lead to an increased risk of adverse drug events as the total number of prescriptions increases. Prescription drug use in the United States has been on the rise. In the year 2022, it is estimated that the number of Americans using prescription drugs will have grown significantly. The reason for the increase can be attributed to a variety of factors, including a growing as well as an aging population, increased awareness and diagnosis of health conditions, and the expanding availability of prescription medications. The United States has seen a steady increase in the number of elderly Americans taking prescription drugs. According to the CDC, the number of Americans age 65 and older taking five or more prescription drugs nearly doubled between 2002 and 2012. CDC estimates that six in ten adults in the United States currently live with a chronic disease such as cancer, heart disease, or diabetes. Over 70 percent of elderly Americans with chronic conditions take at least five prescription medications. Prescription drug use reached a record 194Bn daily doses in 2021. More than 131 million Americans take at least one prescription drugs . Prescription drug misuse has increased by 250% over past 20 years
With the pandemic of Covid-19, an increase in chronic illnesses has been observed. The prolonged physical and mental stress of the pandemic has impacted many people’s overall health, leading to an increased prevalence of chronic diseases and conditions such as Diabetes, Cardiovascular diseases, Respiratory diseases, Depression, Anxiety Stress-related disorders. The increased occurrence of chronic diseases in the US is largely the result of a variety of factors, including the aging population, lifestyle choices such as smoking, drinking, and poor diet, environmental pollution, and lack of access to health care services. Additionally, chronic diseases are being diagnosed earlier and more frequently than before, which has also contributed to their increasing prevalence , and also increase in drug prescriptions. The Covid 19 pandemic has caused an increase in mental health conditions in many countries, which can lead to an increase in the use of prescription drugs. Additionally, many countries have implemented policies to increase access to prescription drugs and mental health services. This increase in access has likely played a role in the increased use of prescription drugs since the beginning of the pandemic. In the US, these increases are particularly prominent among certain groups, including the elderly, the poor, and racial and ethnic minorities. Overall rise in number of drug prescriptions has led to an increase in reports of adverse drug reactions (ADRs)
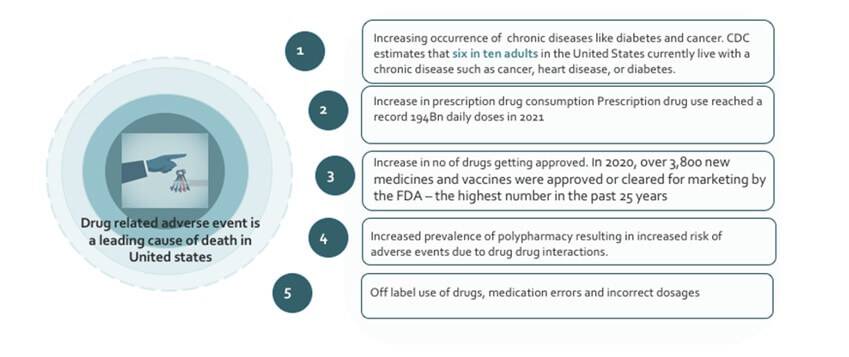
Over the last 10 years, the number of new drug approvals has increased significantly in the United States. According to a report from the USFDA, in 2020, over 3,800 new medicines and vaccines were approved or cleared for marketing by the FDA – the highest number in the past 25 years. Of these new drugs, over 1,900 were approved for the US market. 64% of all patient visits to physicians result in prescription medicines. This increase has been associated with an equally significant rise in the number of adverse events reported in adverse event reporting databases. In addition, the increased number of drugs being approved has also led to the emergence of new drugs and treatments, many of which have been effective in treating conditions for which there was no previous effective treatment. As new classes of drugs and biologics are developed, new risks may emerge that are not seen with existing medications. Therefore, it is important to monitor these new products as they are released and stay current with any new safety information
Adverse drug events (ADEs) are also on the rise due to the increasing prevalence of polypharmacy. Polypharmacy, the use of multiple medications by a single individual, is especially prevalent in the elderly population and can increase the risk of ADEs due to drug-drug interactions (DDIs). Multiple comorbidities in older adults leads to the use of multiple drugs, which increases the risk for adverse drug-drug interactions. Older patients are particularly vulnerable to ADRs because of age-related changes in pharmacokinetics and pharmacodynamics, such as reduced hepatic and renal function, prolonged elimination half-life, and increased sensitivity to drugs which have been shown to be associated with an increased risk of ADRs. However, prescribing drugs to older patients can be difficult because of limited evidence on the benefits and risks of medications in the group. Medical guidelines on medications are usually based on meta-analyses or randomized clinical trials, which can be biased because older adults, particularly those with comorbidity and polypharmacy are excluded from such trials.
As pharmacovigilance becomes increasingly important for understanding and tracking the safety of medications, research is being conducted to understand the extent of DDIs and their effects on patient health. This research is critical for ensuring that the medications used in polypharmacy are safe and effective in combination. As polypharmacy becomes more common, especially in the elderly population, it is necessary to understand the extent and effect of DDIs and their implications for patient safety and health outcomes. Pharmacovigilance can help in the development of strategies to reduce the risks and improve patient safety. To effectively monitor DDIs and assess their impact, it is important to have an accurate understanding of the prevalence of polypharmacy and the number of medications taken concurrently. This data can then be used to create safety and efficacy profiles of commonly used drug combinations. In addition, epidemiological studies can help to identify risk factors associated with polypharmacy and DDIs, such as age, gender, and comorbidities. By understanding the risks, clinicians can make better-informed decisions about medications for elderly patients. Polypharmacy can be caused by a variety of circumstances and healthcare factors, such as:
- Inappropriate prescribing
- Poor drug selection
- Long-term medication use
- Diseases with multiple symptoms
- Co-prescribing of medications with potentially serious drug interactions
Off-label use of drugs, incorrect dosages, and medication errors are all leading causes of adverse drug reaction (ADR) in the United States. According to one study, around 7% of emergency room visits in the US can be attributed to ADRs, with over 50% of those visits associated with off-label use, incorrect dosages, or medication errors. Off-label use of drugs is the practice of using a medication for a purpose other than indications approved by the regulatory agencies. While it is not illegal for a doctor to prescribe a medication off-label, it can increase the risk for adverse drug reactions (ADRs). Till date, few studies have shown a significant association between off-label drug use and adverse drug reactions (ADRs).
Knowingly or unknowingly AI has been creeping into our lives be it in form of Alexa or getting the recommendations on an e – commerce platform. However, suddenly AI became the centrepiece of my life with me joining a Data & AI consulting company Navikenz . With Chatgpt, I am sure AI has become focal point of interest for many others as well. So, I was curious to understand what is AI? How is it different from Human intelligence? How can we possibly marry them both in a manner where both live happily ever after (sounds like a fairy tale even in real marriages😊). With all these questions in mind and after reading varied opinions I thought I should write about my thought process in the simplest manner as there is too much jargon being thrown around. I am not an expert in AI, just trying to learn more about it as it became a front bencher in my life from being a back bencher. So, pls feel free to educate me more on your views.
So, let’s start from the very basic – point zero (though the race to understand AI will not be a finite one)
What is Artificial intelligence?
Artificial intelligence (AI) is a type of intelligence that is artificially created by machines and computer programs. It is designed to simulate human intelligence and perform tasks that would normally require human intelligence, such as problem-solving, decision-making, and language processing.
How is AI different from Human intelligence?
To put it very simply, AI does not have the ability to learn from their experiences or get adapted to new situations or environment in the way that humans can. AI algorithms can quickly analyse vast amounts of data and detect patterns that would be impossible for humans to perceive. For example, AI can process millions of medical images to diagnose diseases with high accuracy or analyse financial data to detect fraud or predict market trends. But humans have the capacity for creative and intuitive thinking, which is still a major challenge for AI.
However, having said that, the next frontier of AI is artificial general intelligence (AGI), which is where computers can mimic how humans learn and exercise cognition in a more general sense. This stage would enable machines to complete any intellectual task that a human is capable of completing. This requires the ability to reason, be strategic, solve puzzles, plan, learn, and make decisions in the face of uncertainty. Scientists believe that we are far from AGI (maybe even 100 years), so let’s focus on AI at the moment.
Current state of AI & it’s impact on businesses
Do we know how AI is going to transform our lives & businesses today? The answer is a resounding – ‘No’. What we are seeing is currently only the tip of the iceberg. We do not know the endless possibilities which AI can open for us. And it’s ok to be scared of the unknown or to have our doubts & raise hard questions about workforce, the legal system, privacy, bias etc. Also, any change in life makes one uncomfortable. Does that mean we become the ostrich and ignore what is hitting us at a rapid pace like never before? The answer is again a resounding ‘No’. AI & human intelligence if combined can provide new insights & perspectives that can enhance human creativity & decision making (their marriage is inevitable). AI has incredible power to transform businesses. In their current state, computers can already ‘see’, ‘hear’ and parse natural language. It has become imperative for businesses to understand what impact AI can have in solving for their business problems. At Navikenz, we can help you answer how AI can help businesses achieve their desired outcomes. Our team is committed to work with you in uncovering & applying the transformative technology to solve for most complex of your business problems in existence today or which might come up in future. Your competition is, if not rapidly at least slowly, accepting AI to be front bencher. So what’s stopping you?
Interested in learning more about AI and its impact on your business? Please email us at [email protected].
150 years ago Jean-Baptiste Alhonse Karr wrote “plus ca change, plus c’est la même chose – the more things change, the more they stay the same…
And so it is with our attempt to put our arms around Enterprise Data in an organization. We go from Data Balkanization to Data Centralization back to Data Balkanization with each iteration promising nirvana but finally realizing that things are back to where we started but faster and with greater ability to know for sure that we are not where we exactly wanted to be.
Data in the 1970/80’s was in ‘flat files’ written by COBOL programs. These were good for running operational applications but completely inaccessible when it came to ‘analysis’. Also each application had its own ‘Data’ in its own ‘files’. So in the 1990’s we moved all these disparate sources of inaccessible data files into huge monolithic ‘Data Warehouses’.
All data from the flat files was poured into structured formats into these Data Warehouses. All the enterprise data was now available ‘in one place’. Except that it was managed by the IT team, with very rigid rules and the sheer size and complexity meant that each time the business wanted any query, they had to wait in queue. Unfortunately, businesses do not run at the speed at which IT (in those days) could react and business domains started creating their own individual small Data Warehouses which were ‘Domain Specific’ called ‘Data Marts’.
These Data Marts were very efficient in serving the needs of the business domain like ‘Sales’, ‘Production’ or ‘Marketing’, but two big problems arose. One is that Enterprise Data started going out of sync. So a Customer name in the Sales Data Mart was now different from the Customer Name in Production and hence there was no way to get a unified view of the Customer, the Product, Sales or anything. So then people started putting complex rules of governance to define ‘Who is a Customer’ and ‘What is a Product’ to be followed by all individual ‘Domains’. But once again business will move at a higher speed than what rules can do to tie it down. The other problem was that while all the above was to do with ‘Structured Data’, it left out the fastest growing volume of new Data which was ‘Unstructured Data’. A Customer ID number with a Customer Name is structured Data. A tweet from a customer complaining about a product is unstructured data but one that just as critically important. So organizations needed to solve for both of the above problems.
Came the Data Lake. Into Data Lakes, just as in the Data Warehouses of old, all the Enterprise Data was poured in, both Structured and Unstructured giving one monolithic place where all the organizational data could be accessed.
We were back to where we started – a monolithic data repository managed by IT which could not be accessed by the business with the ease and speed required.
And by this time Cloud architecture was already there, with scalability and access to storage becoming a non issue. And that has brought us to the latest in this iteration with the Data Mesh architecture, where once again, just like the Data Marts of old, individual business domains are creating their own view of the Data. And thanks to the opportunities afforded by Cloud architecture, unlimited Storage and superfast Computing Speeds, a centralized governance infrastructure of rules surrounding who owns which Data and how to access Data across lines of ownership without having to go back to IT to make massive changes.
That is now a game changer bringing us back to the Balkanized view of data but with a common governance.
So coming up with the Data Architecture is the critical step for an organization that wants to be Digital Leader. We will not solve all the problems, but we will keep going back to the same place but with greater awareness.
What is the right Data Architecture for my organization that will enable me to harness the power of data completely to improve my Digital Maturity. That is the key question for everybody.