Introduction
Did you know?
All vaccines and drugs undergo regressive preclinical and clinical testing for efficacy and safety to get authorized for use.
Medicines and vaccines prevent and treat diseases; however, they also have some undesirable and/or unexpected side effects along with their benefits. It is where Pharmacovigilance comes into action.
The role of Pharmacovigilance is to assess whether the usefulness or effectiveness of a drug to combat diseases outweighs the risks in terms of side effects, serious or non-serious adverse reactions associated with the drug.
Pharmacovigilance is nothing but a drug safety measure that is monitored in the form of adverse drug reactions not only while the drug is under clinical trials but even after it has been approved for market use. In premarket phase drug is under Clinical trials which are conducted under a limited population and on fewer patients. Therefore, it is impossible to uncover all the side effects of the drug as trials do not include high-risk patients, different age groups, and all comorbidity aspects. “Pharmacovigilance is critical in ensuring ongoing safety and efficacy of medicinal products.” Pharmacovigilance is the science and activities relating to collecting, monitoring, researching, assessing, and evaluating information from healthcare providers and patients on the adverse effects of medications and biologics. However, despite all their benefits, evidence continues to show more extensive adverse reactions to medicines that are common yet often preventable and cause illness, disability, and even death. In some countries, adverse drug reactions (ADRs) rank among the top 10 leading causes of mortality. To prevent or reduce harm to any patient, the mechanism for monitoring and evaluating the safety of drugs is vital
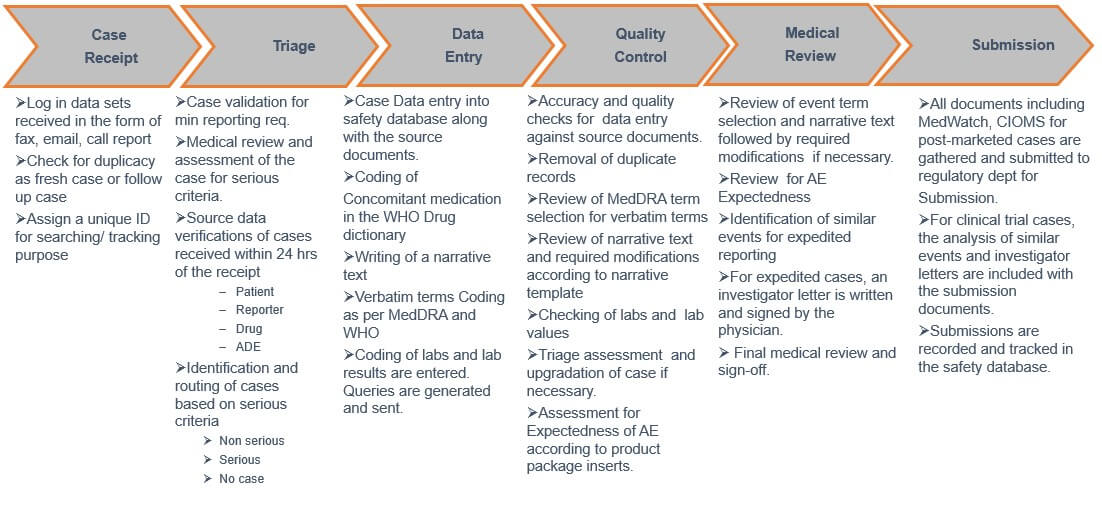
Pharmacovigilance process workflow
Following diagram depicts the typical end to end workflow of the pharmacovigilance process generally followed by pharma companies.
How did the Pharmacovigilance process evolve?
The process of Pharmacovigilance was not formalized until 1960. Then, the tragedy of thalidomide, one of the biggest disasters in the history of medicine, marked the turning point for drug safety evaluation.
The tragedy of thalidomide brought light to many problems and critical issues, in particular, the reliability of animal tests, the behaviour of the industrial company, and the importance of monitoring the drugs after their marketing. In particular, this tragedy evolved the process of Pharmacovigilance.
PV is crucial during the clinical research phase of drug development to ensure it is safe for distribution, but it is also vital to continuously monitor the drug. Post-market safety monitoring is the only way to get an accurate picture of a drug’s safety when the drug is exposed to a much larger population for use and ensure adverse events are reported adequately for review, for instance, through the FDA’s MedWatch gateway.
Major drug withdrawals
Some medicines that were withdrawn from the market and eventually resulted into the evolved Pharmacovigilance process we see in current times.
- Sibutramine – Despite being a helpful drug in obesity treatment, it was withdrawn from the US market in 2010 due to its safety concerns, especially regarding cardiovascular health. Some common side effects of Sibutramine are dry mouth, headache, insomnia, asthenia, obstipation, and sometimes amnesia.
- Valdecoxib – Another drug that was voluntarily withdrawn from the market was Valdecoxib. It was withdrawn in 2005 due to safety concerns of increased risk of CV events and reports of severe and potentially cardiovascular hazards and life-threatening skin reactions, including death.
- Rosiglitazone – The next drug on our withdrawn drug list is Rosiglitazone. This drug got suspended from the market in September 2010 due to the increased risk of ischemic heart disease.
The failure of these drugs raised the importance of giving attention to evaluating study protocols of safety trials before their start.
History And Evolution of Pharmacovigilance
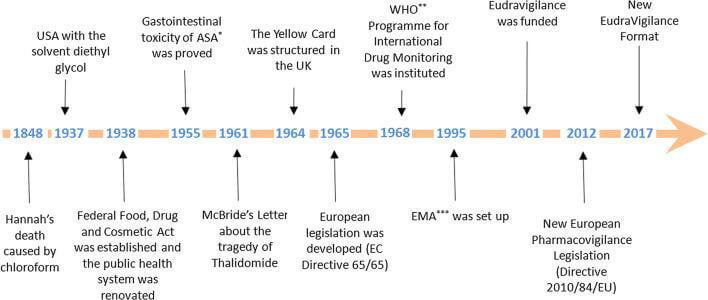
Pharmacovigilance started about 170 years ago but was not named as such at that time. The historical phases and tragedies helped us understand why the pharmacovigilance process is essential to approve any medicine/ vaccination. Let’s have a look at all the milestones that led to the evolution of Pharmacovigilance!
- 1848 – The 15-year-old patient died while performing anaesthesia with chloroform. It caused ventricular fibrillation, leading to the patient’s death.
- 1893 – Lancet initiated a foundation and started collecting information about side effects.
- 1936 – 107 cases after sulphanilamide in the USA.
- 1938 – As per the US Federal Food And Drug Act, “the pharmaceuticals should be pure and free from contamination.
- 1951-1961 – The Thalidomide Tragedy
- 1961 – Over 4000 cases worldwide reported about a 20% increase in fatal abnormalities and phocomelia concerning Thalidomide use.
- 1962 – USA Kefauver-Harris amendment to the law
- 1963 – Resolution WHA 16.36 reaffirmed the need for early action concerning adverse drug reactions.
- 1964 – UK started the “Yellow Card” system
- 1965 – The first European directive – EC directive 65/65 to establish and maintain a high level of protection for public health in Europe.
- 1968 – Pilot Project by WHO was started to gather adverse drug reactions from multiple countries.
- 1997 – ICH E2B adopted the Electronic Reporting standard
- 1999 – Revised MedWatch, draft MedDRA, Institute of Medicine – report on errors and risk issues, Introduction of risk management concepts
- 2001 – Post-marketing safety reporting guidelines by FDA. It highlighted how to report adverse events in the post-marketing phase.
- 2002 – Prescription Drug User Fees Acts (PDUFA III) allowed FDA to charge fees, monitor risk post-approval, and require companies to monitor risks for 2 years post-approval.
- 2004 – Draft risk management guidelines
- 2005 – Final risk management guidelines (specifies how to perform signal detection, risk assessment and risk mitigation)
- 2007 – FDA Amendment Act
- 2008 – Volume 9A in EU
- 2010 – New IND Reporting Rule, European PV legislation passed
- 2011 – Volume 10 (Eudravigilance)
- 2012 – European PV legislation effective (UK SI 2012 No 1916)
- 2014 – MHRA Good Pharmacovigilance Practice for Medicines (Dec 14)
Challenges In Pharmacovigilance
Pharmacovigilance is an essential but challenging process. Some of the challenges in this field are briefly discussed below.
#1. Inconsistent Reporting of Adverse Events and growing volume of adverse events
Adverse events can occur several hours after administering the drug. There are chances that patients might not be able to remember all the side effects and fail to report them accurately. There are chances that patients see symptoms of anxiety as adverse drug effects and report them to their healthcare providers. Sometimes patients fail to follow instructions during medication or experience side effects due to the concomitant drug they’re taking along with the study drug. Such wrong reporting can lead to incorrect conclusions, leading to the suspension or withdrawal of drugs from the market.
The trend for increased reports have been observed for drug products used for prophylaxis and treatment of Covid 19 . The adverse event workload is rising by 50% every year in few pharma companies. This is diluting the focus on serious adverse events needing immediate attention.
As per the Adverse drug event study by Tufts University in Sep. 2015:
- – Underreporting : 40% of HCPs have never reported an ADE
- – Duplicate records
- – 60% of HCPs say it is difficult to determine if the drug has caused the ADE
Solution: Artificial Intelligence in adverse event processing
Although the US FDA is broadly exploring the use of AI for PV, we focus on the application of AI to the processing and evaluation of Individual Case Safety Reports (ICSRs) submitted to the FDA Adverse Event Reporting System (FAERS). We can easily integrate AI into some aspects of ICSR processing and evaluation, but the performance of current AI algorithms requires a ‘human-in-the-loop’ to ensure good quality. However, some outstanding scientific and policy issues should be addressed before the full potential of AI to exploit for ICSR processing and evaluation, including approaches to quality assurance of ‘human-in-the-loop’ AI systems, large-scale, publicly available training datasets, a well-defined and computable ‘cognitive framework,’ a formal socio-technical framework for applying AI to PV, and development of best practices for integrating AI to PV. Nevertheless, we believe that the stepwise implementation of AI for ICSR processing and evaluation provides a foundation for the further development of AI approaches to other aspects of PV.
#2. Spontaneous Reporting Issues
Anyone can report the adverse events – patients, companies, or the HCP. However, there are chances of under-reporting adverse events in the post-marketing databases. Sometimes, the medical staff does not prioritize reporting and may neglect symptoms that are not serious. It can happen due to the workload increase on medical staff. Another concern is the misreporting and miscoding of adverse. For example, the fields in reports about dosage, formulation type, time, and length of exposure to adverse events are not clearly reported and coded, leading to challenges in managing and analysing the data. It also leads to the generation of false alarms for non-existent adverse events. HCPs generally select treatments based on their practice preferences; also, the inadequately adjusted algorithms could produce errant false signals. Inadequate analysis of such signals may lead to early refusal of valuable drugs.
Solution:
The potential solutions suggested for improving spontaneous reporting were to define the kind of ADRs which should be reported:
- – To facilitate easy contact and quick access to the hospital pharmacovigilance system,
- – To enable information and support for reporting and feedback on pharmacovigilance activities.
#3. Analysis of Electronic Health Records (EHR)
EHR provides a great wealth of information about real-time and real-world medication usage. However, a few limitations include the unstructured narrative information that is complicated to analyse. In addition, the medical industry collects a dazzling array of data daily, making it difficult for professionals to analyse the data and raise signals.
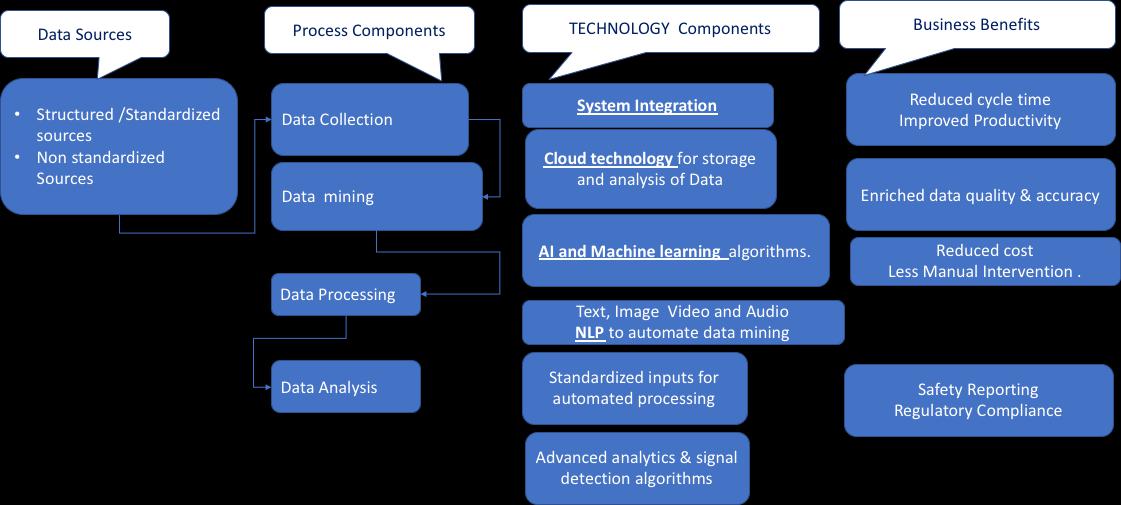
Solution: Data Mining
Data Mining on Electronic Health Records has emerged as a promising solution in Pharmacovigilance. As we know, gaining insights about any particular drug requires a lot of data. Gathering this data requires a lot of time and effort. Data Mining can be used to review many reports with the NLP technique. Moreover, advanced surveillance systems can be integrated into the PV system to identify drug safety signals and facilitate reporting of suspected adverse drug reactions.
#4. System Integration
The pharmaceutical industry relies on various systems, including clinical trial management systems (CTMS), clinical data management systems (CDMS), product performance systems, clinical coding applications, and CRO systems for pooled data analysis. Moreover, signal definitions, medical domains, medical coding’s, and adverse events must be appropriately standardized for signal analysis. However, standardization is the biggest challenge in Pharmacovigilance as there is no standard framework to allow system integration. Though the agreed file format to be used is XML; however, it is not implemented.
Solution: Blockchain
This is where blockchain comes into play. Blockchain pharmacovigilance deals with the detection, medical assessments, evaluations, monitoring, and prevention of ADRs. The apparent increase in ADRs data volume has put a lot of pressure on pharmaceutical companies to maintain records. Due to this, pharma companies are integrating Blockchain-based PV solutions to ensure drug monitoring and safety. Blockchain provides a decentralized system and evenly distributed database in terms of nodes, helping companies to fetch real-time data with just a single click.
Some other benefits of using blockchain pharmacovigilance:
- – It helps create an appropriate reporting structure to assess and monitor drugs in clinical trials and post-approval.
– It helps create appropriate protocols for drug recalls, issuing warnings to patients/customers, and assures compliance.
– It creates and provides better assessment and evaluation for the drugs to ensure long-term viability for business and gain the trust of customers/ patients.
Conclusion
As you’re aware that PV ensures whether a drug works and is safe to use throughout the drug lifecycle, every pharma company needs to set up a pharmacovigilance system to ensure drug safety and efficacy. However, setting up a pharmacovigilance system requires expertise in analysis, data collection, risk management planning, and writing/reporting standards. Moreover, you’ll need to invest in a safety database and hire qualified experts with knowledge of the local language, regulations, and resources. These experienced PV service providers will help you meet pharmacovigilance requirements for your market authorization applications.
Strategy is about making choices. Choices about “Whom to serve?”
A Customer is not someone who gives you business. A Customer is that person whom you choose to receive business from. And then be the best in serving them with your product or service. You must ‘Choose’ your customer.
Every company has a lot of internal problems. The best way of solving all these problems is to ‘fire’ all of your customers. But of course you can’t do that. But you might find that 60% of your customers? How much of your problems will you solve? 60% of your problems – probably more. But of course we need ‘new’ customers today who will be our anchor customers tomorrow.
- Don’t have time: We have the right customers if only we can execute and deliver: When a company is bogged down by bad delivery, more often than not the reason is trying to serve the incorrect customer segment, one that you are not structured to excel in. But Management Loves ‘Busyness’ and everybody is busy spending time on ‘solving problems’ as opposed to trying to understand the root cause of the problems. “Hence I don’t have time for Strategy.”
- Nobody Understands my problems: On a daily basis there are issues with customers, suppliers, finance, HR, prospective employees, legal. Solving each of them requires a deep understanding of the unique situation and culture of the organization. Nobody can help without a deep understanding of all these interdependencies. And all current people are busy solving these problems. “If only we can overcome the current situation then we will be OK.”
- That will not happen. Typically, a company’s ‘Strategy’ should be in alignment with its ‘Structure’ and how it manages its ‘Execution’ wrapped in a harmonious ‘Culture’. In most companies there is a gap in the alignment of Strategy with Structure, its Culture and its Execution. But to management which is sitting on the boat this can be less obvious. To management “My situation is unique and nobody from outside can understand what I am going through.”
- Don’t have the people: Focusing on understanding Strategy requires people different from managers who are running the company. These people typically should not have a deep involvement in ‘current’ execution but have a wider view of other companies across industries. These people cannot be found within the company and even if they exist, they will be the typical ‘High-Po’ (High Potential) people who cannot be excused from current assignments. Hence the people to do this are not there internally. For management “the people who can help me do this are insanely busy on critical initiatives right now.”
- It is Expensive: Yes it is always expensive to hire some people from outside to do ‘Strategy’. But typically that is like directing a ‘Firehose’ to water a dry flower-pot. The strategy team will come in for a few weeks and provide a ‘deluge’ of best practices that is way beyond the capability of the company to absorb in that short amount of time. And later any lack of improvement in performance will be due to the company’s inability to ‘implement’. “I cannot spend a million dollars for a few weeks of strategy engagement that I am not ready to absorb the results of. It will also take time away from my current priorities”.
But fundamentally the importance of ‘Strategy’, where the key question is asked and answered remains: “Who is my customer and Why?” Inability to get something done does not remove the need for it. In fact, makes it even more urgent to act upon.
“What do you think?”
Yes. What do you think is one of the most powerful weapons in a Leader’s arsenal. But very frequently leaders either fail to deploy it or don’t know when and how to use it.
As a percentage of time, very little of the time in a day is when a leader comes across good news. Mostly it is issues, turf wars, disgruntled employees or customers, missed commitments from others that affect your company, unforeseen breakdowns etc. And that goes on relentlessly day after day. And many a times, in fact most often, a leader’s instinct is either to ‘find somebody to blame’ or to be the ‘leader’ and come up with the ‘right’ approach to the situation.
Both the approaches are severely flawed.
It is easy to find somebody to blame and analyze the situation so that ‘we learn a lesson’. But that is flawed as the easiest way in which people will not make any mistake is if they don’t do anything. Particularly anything that is new and innovative and anything that has even the slightest chance of failure. So if people feel, and even if one person is blamed for a ‘mistake’ the entire organization will learn very quickly from the lesson, people across the organization will learn that taking a risk is not a good idea and the best way to survive in the company is to ‘keep your head down’.
What a leader should do is focus completely on solving the situation by harnessing the power of the collective. If you found somebody to blame, then it becomes their job to solve the problem. Everybody else might take pity and ‘help’ this person get out of the hole, which will further ensure that the person will only feel more bitter and in turn blame everybody else for creating circumstances that forced him/her to make the purported mistake. They will not accept it as their problem. So focus completely on bringing everybody to solving the problem and unleash the most powerful weapon. Ask them “What do you think” we should do.
And ask the question without judgement, with openness and with an eagerness to learn. Chances are in this situation the person who was the proximate cause will say “I thought I messed up” and others will say “I could have done ‘a’, ‘b’ or ‘c’, to help the person so that we did not come to this situation”. The collective will come together to solve the problem. And the great thing is that you as a leader will just have to lean back and see it all unfold.
Similarly, much more often than it should be, a leader wants to show that s/he is the leader and has the answers to the problem. You don’t need to have the answers. More powerful the leader more aware they are that better answers are with other people. Their power as a leader is to create the environment to harness those. In almost any type of situation taking the time to ask “What do you think?” can be a powerful door opener to avenues of thought you never expected. And this could be deployed in almost any sphere as long as it is asked for in the right manner:
“Our sales in Australia have now declined for two quarters in a row, the sales team there is arrogant and and the presales organization does not want to work with them apart from a general weakness in demand in Australia. Should we change the sales leadership there?
“What do you think we should do?”
“We have this new product that we need to invest in but there is no budget for it this quarter. However, there is a running Proof of Concept that has been going on for six quarters now and although the engineering team is very excited, the prospects from sales look much bleaker than we had originally anticipated. Customers seem happy to engage in a POC but we can’t figure out how to monetize it. We can save some money from there and deploy it for this new idea. What should we do?
“What do you think?”
“We had a great year and the entire quality team really performed to meet the increased volume with no let-up in standards. The time for promotions and increments has come and if we recognize only a few and not the entire team it will break the tremendous sense of ‘teamwork’ with which the whole group has worked this year. What should we do here? Promote the entire team or keep them all back so that the team is together?
“What do you think?”
And once you get the answers, the leader absolutely does not need to respond with ‘Let me now teall you what I think?” Leader just needs to absorb for further thought and ask the person to go back and think some more and suggest on “What we should do”. 99% out of 100% the problem will solve itself. The leader will have to not do anything.
But as leaders, our predetermined proclivity is to ‘do something’. Don’t.
Ask “What do you think?”
In increasingly competitive environments, digital technology is a fundamental element that enables and supports a company’s short and long-term business objectives for a specific market or an entire industry.
The introduction, implementation and adoption of innovative digital solutions must be planned and executed in an organized manner. Essentially, a company’s ability to deliver outstanding products and services, while remaining relevant, competitive and profitable, depends on it.
As such, technology-driven companies should have a process to identify, rationalize, incubate and manage effectively new technologies and solutions. The outcome of that process is a Digital Technology Roadmap. It includes the necessary coordination and the key milestones to meet a company’s objectives through the adoption of digital technologies and solutions.
If selected discretely, digital technologies may bring inconsistencies (potentially affecting the quality and reputation of products and services), induce redundant capabilities (stretching teams too thinly or increasing staff dramatically), and generate unnecessary costs (impacting business cases and profit margins). An Enterprise-wide approach and roadmap, along with a mindset of cross-functional collaboration, engineering excellence and customer centricity, will alleviate this problem.
Regular reviews of the Enterprise Digital Technology Roadmap will ensure that key IT and non-IT stakeholders align on the overall strategy, priorities, dependencies, gaps and investment decisions. They will also facilitate the timely allocation of internal staff and engagement of IT partners. As importantly, an Enterprise Digital Technology Roadmap will also provide a general sense of direction that can be used internally as a source of motivation, and also externally as a marketing tool.
Formulating and executing an impactful roadmap, one that effectively enables digital innovation and realizes the full potential of the organization and its assets, is no easy task. Too often, because of operational imperatives, companies are not able to invest the time or do not have the capacity to analyze their current situation, envision the future holistically, and drive strategic initiatives. Navikenz has the experience, insight, transformation framework and passionate people to identify the various areas for improvement, build a roadmap and game plan, organize the necessary changes, and manage your transformation programs.
Navikenz is here to help. Contact us.
The CEO of a midsized company once remarked “my employees line up at the exit door just before 5 PM. It is almost an exodus as soon as the clock strikes five”. What is going on? And this of course was before the pandemic when people came to work. How is this connection frayed even further when people are virtual? What is the solution?
The company is doing well, is profitable, respected in its segment and has been growing fairly consistently over the last 30 years. But is it growing faster than its competitors, is its profitability higher than the industry average and does it have employees who are actively sought after by other companies?
Every employee brings their mind and their body to work. That is why work gets done, checklists get ticked off, customer orders met, and money collected. These are table stakes. Every company does this. But which are the other companies where the employees give that extra 5% in their efforts which results in dramatically improved performance for the company at large. It is those companies where the employee is bringing not only their mind and their body to work but also their soul.
And management’s job is to create the necessary conditions for people to be able to contribute with their mind, their body, and with their soul. And that happens when work is not about meeting checklists and deadlines but about a larger purpose. Everybody working at the company should not only believe that they are serving customers and getting paid, but also fulfilling a critical part of making the world a better place. And while doing so, they are also learning and growing.
Management’s job is not to provide employment but to provide employability in which the people continuously become better than their own selves, and hence contribute to making the company better in the process. Every employee has options. They should be eminently employable in other companies but choose to continue working in your company. Your job is to give them that purpose and that environment which enables that 5% extra performance in a continuously learning organization.
That is when they will contribute to success with their mind, their body, and with their soul.
David Ricardo, the famous British Economist, in his book ‘On the principles of Political Economy and Taxation’ published in 1817 introduced the theory of ‘Comparative Advantage’ of nations and the reason why trade was meaningful for all countries. He said that by definition every country has a relative advantage in some ‘goods’ and it makes sense to trade for that with other countries. In fact every country has an ‘Absolute Advantage’ and uncontested superiority to produce a particular good better than any other country.
What is the ‘uncontested superiority of a company to produce a good better? That is the ‘Competitive Advantage’ of the company. Do we know it? Do we recognize it and work towards strengthening it?
In the case of nations, if one nation has high unemployment and cannot manufacture goods due to low capital, by definition it must have low labor costs that it can exploit with the right governing infrastructure.
Similarly, if a company produces low volume widgets and is getting killed on price by high volume widget manufacturers, it can either improve its volumes to reduce its costs of production to compete (and thereby move into the ‘Red Ocean’ of intense competition) or deeply analyze its own business to find its own unique competitive differentiator that is available only to that company and improve that (and find its own ‘Blue Ocean’ for growth).
The driving axiom is that every company has a unique competitive differentiator that is available only to that company and ‘nobody else has it’. The call for management is to think deeply through their business and find that ‘unique’ differentiator. Finding that differentiator has two vectors, the services or products that you sell, and to whom you sell it to. At the intersection of the two there is a unique position that is open only to you.
To give a plebian example, you cannot be the best Hamburger maker in the world. But you can absolutely be the best Hamburger maker after crossing the bridge and before the town center (where you want to play) that serves Korean Hamburgers and has a driver through (doing things differently). Absolutely no reason why you cannot have an absolute advantage as this Hamburger place. Now, can you have enough volume of business to justify the segment selected is a separate analysis.
But every company must find its own unique Blue Ocean, it own Absolute advantage where it has uncontested superiority in what it does for its customers.
What is your ‘Uncontested Superiority?’ What is your ‘Competitive Differentiator?’
Most companies in the services business get to a point in their journey where they realize that the key to growth is to get a few large deals. Smaller contracts are good to provide a steady and diverse base of operation. But to achieve significant growth and momentum, services companies need to have one to two large jumps, every year. These jumps may come organically through a commercial contract of significant value, or inorganically with a corporate action like an acquisition or a merger.
Large deals, of any kind, can be double-edged swords. They lift the company operations to new heights but also can create significant flux and distraction within the organization – both in terms of the company’s operations, but also its basic culture and structure. Without careful handling, they can create so much disruption that they endanger the whole ship.
Preparing for the acquisition
When the deal is related to an inorganic move, then the implications are even larger. Information regarding an inorganic deal in the works, is usually limited to a small audience within the company – the Board, CEO, and CFO are usually involved, but beyond them, only a very small set of people are ‘in the know’. This means that once the rubber hits the road, the organization is left with very little time to prepare itself. Senior management within the business will look towards the CEO and CFO to guide them on how to deal with the transition and protect the culture and operations.
Unfortunately, senior leadership becomes too busy doing the negotiations for the acquisition. They do not take the time to step back and think about how they will guide the organization through the transition. As a result, after the deal is announced, managers get mixed messages from the leadership which causes confusion and consequently the organization loses momentum.
As an organization considering a large deal, it is important for the CEO to take the following steps.
1. Ensure there is a common consensus on the strategic rationale:
The primary role of the CEO and CFO is to set the direction and define the boundaries. Have a clear rationale for the need of the deal/acquisition, and what it brings to the table. Be aware of the boundary conditions beyond which the deal should not be done. Have a common view of what the ideal outcome would look like.
2. Have clarity on what the long-term objectives are
Ask what the company will gain post the deal, beyond just the increase in overall revenue.
a) Are there synergies that can be tapped? Will there be cross-selling possibilities? b) Does it open other opportunities that were previously unavailable?Calculate the value of each opportunity and then reduce the overall number by a factor to account for internal inefficiencies.
3. Actively evaluate the risks:
Build scenarios that deviate – positively and negatively – from the planned outcomes. Both deviations are important. Identify the key inputs that have the greatest impact on the deal outcomes and run a sensitivity analysis for each. Identify who holds the contractual risk, and ensure the company is protected against the greatest risks. Consider the risk of cultural misalignment, especially when the entities in question are intended to be integrated together.
3. Create an outline of the 30, 60 and 100-day plans, ahead of deal announcement:
Use this plan to set the tone as soon as the deal is announced. Ensure that critical deal objectives are converted into milestone-based outcomes within the plans.
5. Invest in a resource that can take ownership of the deal-making process:
Appoint a talented manager from the ranks to manage the deal process. The ideal person should understand finance, but in a pinch. A good project manager with limited financial understanding is better than a finance expert who cannot drive the integration process effectively. Ensure the manager is senior enough to make decisions, but junior enough that they can be pulled out of their day job to focus on the acquisition full-time.
In conclusion…
Deal making is game-changing, and exciting. But behind the glamour of signing that multi-million-dollar contract, there is an immense amount of serious planning effort. Only those leaders who invest time and energy to do the thinking work can enable their organizations to reap above – average benefits.