
Drug related adverse event is a leading cause of death in the US

Balaji Krishnan
Chief of Customer Success
Recent trends in drug safety and pharmacovigilance have revealed that drug related adverse events are the major cause of death in the United States. Every year, an estimated 200,000 to 400,000 people die due to adverse drug reactions, which is more than stroke and diabetes combined. Approximately 2.7 million adverse drug reactions occur annually in the US, resulting in over 100,000 hospitalizations and more than 15,000 deaths. This makes ADRs the fourth most common cause of hospitalisation in the USA, behind heart attacks, stroke and pneumonia. There are several factors that contribute to America's growing number of hospitalisations and deaths related to adverse drug reactions. These primarily include, poor patient compliance with medication instructions , lack of education on potential side effects , unnecessary prescribing of medications and increased use of polypharmacy, or combining multiple medications
What has changed ?
However in recent years the space of Pharmacovigilance been changing rapidly due to advances in technology and the influx of data. In the past five years, there have been many changes in the way the field is handled. As the range and scope of drugs being approved and being used expand, monitoring the safety of medicines and ensuring that they are being used correctly and as directed is becoming an increasingly complex task. With more and more number of drugs being available, pharmacovigilance practitioners must remain on top of the latest developments in order to ensure that patients are receiving the safest and most effective treatments available.
The total number of prescriptions dispensed in the United States increased from 3.9 billion in 2007 to 4.3 billion in 2012, according to the Centres for Disease Control and Prevention. This represents an increase of 11%. This can lead to an increased risk of adverse drug events as the total number of prescriptions increases. Prescription drug use in the United States has been on the rise. In the year 2022, it is estimated that the number of Americans using prescription drugs will have grown significantly. The reason for the increase can be attributed to a variety of factors, including a growing as well as an aging population, increased awareness and diagnosis of health conditions, and the expanding availability of prescription medications. The United States has seen a steady increase in the number of elderly Americans taking prescription drugs. According to the CDC, the number of Americans age 65 and older taking five or more prescription drugs nearly doubled between 2002 and 2012. CDC estimates that six in ten adults in the United States currently live with a chronic disease such as cancer, heart disease, or diabetes. Over 70 percent of elderly Americans with chronic conditions take at least five prescription medications. Prescription drug use reached a record 194Bn daily doses in 2021. More than 131 million Americans take at least one prescription drugs . Prescription drug misuse has increased by 250% over past 20 years
With the pandemic of Covid-19, an increase in chronic illnesses has been observed. The prolonged physical and mental stress of the pandemic has impacted many people’s overall health, leading to an increased prevalence of chronic diseases and conditions such as Diabetes, Cardiovascular diseases, Respiratory diseases, Depression, Anxiety Stress-related disorders. The increased occurrence of chronic diseases in the US is largely the result of a variety of factors, including the aging population, lifestyle choices such as smoking, drinking, and poor diet, environmental pollution, and lack of access to health care services. Additionally, chronic diseases are being diagnosed earlier and more frequently than before, which has also contributed to their increasing prevalence , and also increase in drug prescriptions. The Covid 19 pandemic has caused an increase in mental health conditions in many countries, which can lead to an increase in the use of prescription drugs. Additionally, many countries have implemented policies to increase access to prescription drugs and mental health services. This increase in access has likely played a role in the increased use of prescription drugs since the beginning of the pandemic. In the US, these increases are particularly prominent among certain groups, including the elderly, the poor, and racial and ethnic minorities. Overall rise in number of drug prescriptions has led to an increase in reports of adverse drug reactions (ADRs)
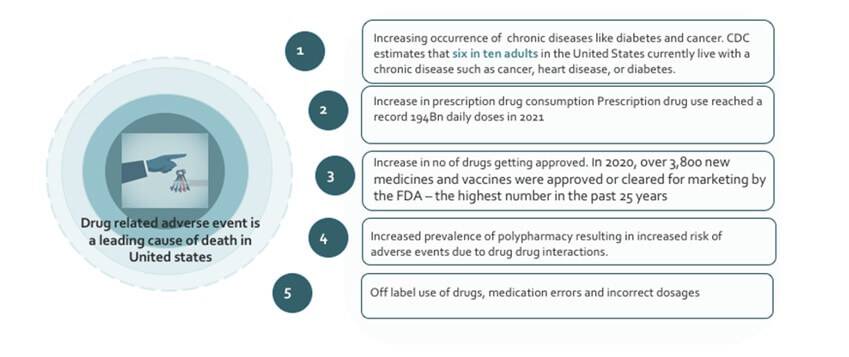
Over the last 10 years, the number of new drug approvals has increased significantly in the United States. According to a report from the USFDA, in 2020, over 3,800 new medicines and vaccines were approved or cleared for marketing by the FDA – the highest number in the past 25 years. Of these new drugs, over 1,900 were approved for the US market. 64% of all patient visits to physicians result in prescription medicines. This increase has been associated with an equally significant rise in the number of adverse events reported in adverse event reporting databases. In addition, the increased number of drugs being approved has also led to the emergence of new drugs and treatments, many of which have been effective in treating conditions for which there was no previous effective treatment. As new classes of drugs and biologics are developed, new risks may emerge that are not seen with existing medications. Therefore, it is important to monitor these new products as they are released and stay current with any new safety information
Adverse drug events (ADEs) are also on the rise due to the increasing prevalence of polypharmacy. Polypharmacy, the use of multiple medications by a single individual, is especially prevalent in the elderly population and can increase the risk of ADEs due to drug-drug interactions (DDIs). Multiple comorbidities in older adults leads to the use of multiple drugs, which increases the risk for adverse drug-drug interactions. Older patients are particularly vulnerable to ADRs because of age-related changes in pharmacokinetics and pharmacodynamics, such as reduced hepatic and renal function, prolonged elimination half-life, and increased sensitivity to drugs which have been shown to be associated with an increased risk of ADRs. However, prescribing drugs to older patients can be difficult because of limited evidence on the benefits and risks of medications in the group. Medical guidelines on medications are usually based on meta-analyses or randomized clinical trials, which can be biased because older adults, particularly those with comorbidity and polypharmacy are excluded from such trials.
As pharmacovigilance becomes increasingly important for understanding and tracking the safety of medications, research is being conducted to understand the extent of DDIs and their effects on patient health. This research is critical for ensuring that the medications used in polypharmacy are safe and effective in combination. As polypharmacy becomes more common, especially in the elderly population, it is necessary to understand the extent and effect of DDIs and their implications for patient safety and health outcomes. Pharmacovigilance can help in the development of strategies to reduce the risks and improve patient safety. To effectively monitor DDIs and assess their impact, it is important to have an accurate understanding of the prevalence of polypharmacy and the number of medications taken concurrently. This data can then be used to create safety and efficacy profiles of commonly used drug combinations. In addition, epidemiological studies can help to identify risk factors associated with polypharmacy and DDIs, such as age, gender, and comorbidities. By understanding the risks, clinicians can make better-informed decisions about medications for elderly patients. Polypharmacy can be caused by a variety of circumstances and healthcare factors, such as:
- Inappropriate prescribing
- Poor drug selection
- Long-term medication use
- Diseases with multiple symptoms
- Co-prescribing of medications with potentially serious drug interactions
Off-label use of drugs, incorrect dosages, and medication errors are all leading causes of adverse drug reaction (ADR) in the United States. According to one study, around 7% of emergency room visits in the US can be attributed to ADRs, with over 50% of those visits associated with off-label use, incorrect dosages, or medication errors. Off-label use of drugs is the practice of using a medication for a purpose other than indications approved by the regulatory agencies. While it is not illegal for a doctor to prescribe a medication off-label, it can increase the risk for adverse drug reactions (ADRs). Till date, few studies have shown a significant association between off-label drug use and adverse drug reactions (ADRs).