
Unveiling the Hidden: Harnessing Big Data for Drug Safety Signals

Balaji Krishnan
Chief of Customer Success
In the pharmaceutical industry, it is essential for professionals to keep themselves informed about current regulations and best practices in managing drug safety signals. This is vital to safeguarding the well-being of patients and meeting regulatory requirements. As technology and data continue to advance, pharmacovigilance is becoming increasingly intricate and advanced. It is wise to allocate resources towards implementing strong systems and procedures to remain ahead in this evolving landscape.
A signal is a piece of information or data collected from various sources that reveals something new about a drug intervention, a drug-related event, or a previously known event. This information can indicate a potential connection between the drug and the event, whether it is negative or positive. It is important to understand that a signal does not prove a direct cause-and-effect relationship between a side effect and a medicine. Instead, it serves as a hypothesis that requires further evaluation based on data and reasoning.
These signals can suggest previously unknown side effects of a drug or indicate that the risks of the drug outweigh its benefits. Safety signals can originate from various sources, such as reports from patients and healthcare providers, clinical studies, and post-marketing surveillance. It is essential to identify and closely monitor these signals to ensure the safety and effectiveness of drugs.
In the modern era of digital technology, a tremendous volume of data is gathered daily. This data can be analysed to uncover fresh insights and knowledge that aid in making well-informed decisions. The healthcare sector is no stranger to this phenomenon. With the emergence of electronic health records and other digital health technologies, researchers now have access to extensive patient data. One area where this "big data" approach holds great promise is drug safety monitoring. In this article, we will explore how big data can be utilized to identify signals related to drug safety and the potential advantages it offers for ensuring patient well-being.
Signal Management
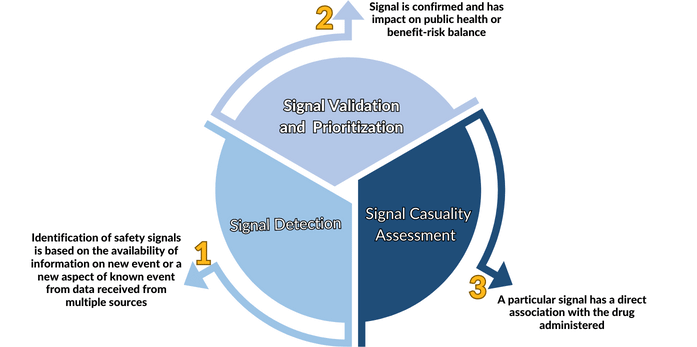
Effective signal management is critical for the safety of patients throughout the product lifecycle. It involves a systematic approach to identifying, evaluating, and responding to signals of potential safety concerns, including adverse events and other safety data. By following these sub-steps, stakeholders can ensure that signals are properly identified, evaluated, and acted upon in a timely manner. The concept of drug safety signal is not new. It has always been the cornerstone of pharmacovigilance. As the number of approved drugs and the prevalence of individuals taking multiple prescription medications continue to rise, there has been a corresponding increase in the reporting of adverse events. However, here are a few things to consider regarding adverse events and signals:
- Not all adverse events meet the criteria to be classified as signals. Certain adverse events are anticipated and do not necessitate additional investigation.
- A signal is usually described as information that indicates a connection between something we did and something that happened. It could be a new connection we did not know before, or a new aspect of a connection we already knew about.
- If certain conditions are met, some adverse events can be considered signals. These conditions may include the events being of high severity or unexpected in nature.
- Deciding whether an adverse event is a signal or not involves carefully looking at the data and information available. The process involves gathering data, identifying signals, confirming, and categorizing them, giving priority to important signals, assessing them, and effectively managing the associated risks.
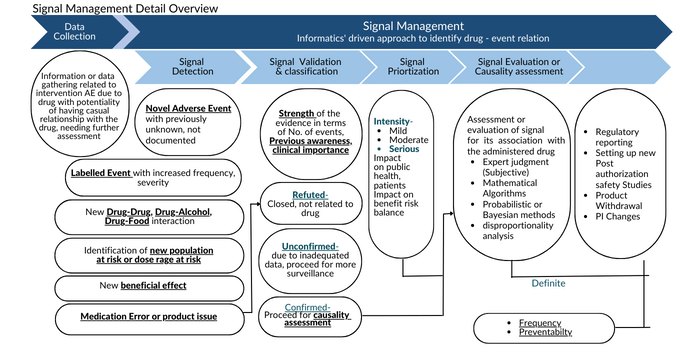
Data Collection
Data or information concerning any intervention involving a drug that may have a cause-and-effect relationship requiring further examination is gathered. This data can come from various sources, such as requested reports, unsolicited reports, contracts, and regulatory authorities. In recent times, statistical methods have been developed to analyse this extensive amount of data. These methods, known as data mining techniques, are preferred over traditional methods to ensure early detection of signals from large and complex data sources.
Signal Detection
When a drug is created, it is intended to have a specific effect on a particular part of the body. However, drugs can also affect other parts of the body. These effects can be helpful or unwanted. For example, antihistamines like cetirizine can provide relief for cold or allergy symptoms but may also cause drowsiness. Medications can have both desired effects and undesired effects, which are called adverse drug reactions. Adverse drug reactions can happen from taking a single dose of a drug or using it for a long time, or when multiple drugs are taken together. On the other hand, adverse events are unexpected medical events that occur in patients who have taken a medication, but these events may or may not be directly caused by the medication.
According to the definition by WHO-UMC, a safety signal is information about a potential side effect of a medicine, whether it is already known or newly discovered. This information is usually based on multiple reports of suspected side effects. It is important to understand that a signal does not prove a direct cause-effect relationship between a side effect and a medicine. Instead, it serves as a hypothesis that requires further investigation based on available data and arguments. Signals can bring new insights or confirm existing associations between a medicine and an adverse effect. As more data is gathered, the information in a signal may change. To evaluate the relationship between a medicine and a side effect, a causality assessment is conducted.
Signal Validation and Classification
During signal validation, the data supporting a detected signal is carefully examined to confirm the presence of a new potential cause-and-effect relationship or a new aspect of an existing relationship. Once this evaluation is done, the signal can be categorized as validated, refuted, and closed, or monitored. Various factors are considered to determine the validity of a signal, such as the strength of the signal, the timing of events, the presence of risk factors, the source of data, the relationship between the dose and the signal, the consistency of reported cases, and the connection between the reaction and the medication on a pharmacological or biological level.
Strength of the Signal
In most cases, there is a clear connection between the occurrence of the adverse reaction (including the first signs or symptoms) and the administration of the suspected medication.
Some clinically relevant cases have shown positive outcomes when the medication is temporarily stopped (de-challenge) or resumed (re-challenge) with appropriate time intervals. These cases increase the likelihood of a relationship between the adverse event and the drug.
A substantial number of cases that do not have information on de-challenge or re-challenge outcomes do not present any risk factors such as concurrent conditions, other medications, medical history, or demographics. This further supports the possibility that the event is due to the administered drug.
The signal is identified by examining significant findings from reported cases, both solicited and spontaneous, as well as from scientific and medical literature. Similar findings are also observed in the literature for drugs belonging to the same category.
The reported cases consistently demonstrate a connection between the dosage of the medication and the observed effects. These cases also display a consistent pattern of symptoms, supported by multiple sources of evidence. There is a clear cause-and-effect relationship between the adverse reaction and the administration of the suspected medication, considering its pharmacological, biological, or pharmacokinetic effects. The reported signs, symptoms, diagnoses, and tests conducted align with recognized medical definitions and practices.
In all the above scenarios, the signal is considered valid.
Clinical Relevance of the Signal
To respond effectively, it is important to grasp the clinical significance of signals. Knowing the potential risks linked to medication use can contribute to patient safety and better health outcomes.
Signals hold clinical relevance when they involve life-threatening conditions, necessitate hospitalization and medical interventions, have a significant number of reported deaths or disabilities unrelated to the treated disease or existing health conditions, affect vulnerable groups or individuals with pre-existing risk factors, exhibit patterns related to drug interactions or specific usage patterns, and influence the balance between the risks and benefits of the suspected medication.
Once a signal is confirmed as valid, it undergoes additional monitoring and investigation to determine its exact relationship with the medication that was administered. This process is called causality assessment or signal evaluation. Meanwhile, if the event is serious, it may be reported promptly while waiting for the results of the causality assessment.
If the analysed information does not support a validated signal, it is considered closed or refuted. This can happen if it was a false alarm, there is insufficient evidence to continue monitoring, the signal is no longer relevant due to discontinuation of the drug or lack of evidence of a genuine safety concern, or further monitoring is necessary to ensure ongoing safety.
Classifying drug safety signals as closed, refuted, or validated is an important task in identifying potential negative events associated with medications. Several methods, including machine learning algorithms, have been suggested to automate this process, but their accuracy is still being evaluated. Standardized approaches are needed to enhance the classification of drug safety signals.
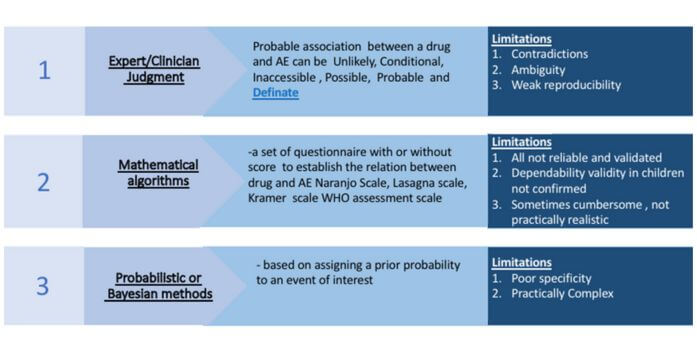
Causality Assessment
Causality assessment is the process of examining the connection between a signal and the drug that was given. The Naranjo scale, a commonly used tool in pharmacovigilance, helps assess causality by considering factors like the timing of events, the dosage of the drug, information about stopping and restarting the drug, and other explanations for the observed effects.
Risk Management
During the process of causality assessment, if a link between the signal and the drug is confirmed, regulatory agencies carefully evaluate the situation to decide if additional measures are required. These measures can involve updating the information and labelling of the product, withdrawing the drug from the market if necessary, and planning studies to ensure safety across diverse groups of people. Risk management plans are crucial to prevent future incidents by identifying potential risks, creating protocols to respond to safety signals, and regularly reviewing and updating the plan.
The main objective of managing drug safety signals is to protect patients from harm and ensure that safe and effective drugs are accessible to the public.